Dear Members,
A big thanks to all of those who participated in one of the first community-based testing programs of an outpatient population using fingerprick antibody assays in the US. Due to a sudden drastic change in FDA regulations made last week, fingerprick antibody testing is now only allowed to be performed by academic, research, and hospital-based laboratories with a level 3 CLIA certification (until the FDA changes the statutes again). So unfortunately we are no longer able to offer this service at Cloud.
One of our original goals was to prove whether such population testing programs can feasibly be conducted in the primary care setting. It was certainly not a simple undertaking but we felt is was very important for myriad reasons. We still have no idea what the prevalence of COVID19 is in our community and we hope that, as our data is carefully analyzed over the coming week, we will be able to provide at least some insights as well as comment on the utility and efficacy of these particular diagnostic tools.
Prior to the FDA restriction we’d tested nearly 800 individuals, and over 100 have tested positive for one or both antibodies. We are now in the process of confirming those who tested positive with a quantitative blood draw antibody test (which fortunately is still allowed by the FDA). Our aim is to anonymize all of our data, re-check and confirm all of the results, analyze them using the best statistical methods available, and publicly release this data to anyone interested in seeing it.
We are extremely proud and grateful to everyone who supported this endeavor, most of all our amazing staff who went above and beyond under challenging circumstances and time constraints. Thanks to those who contributed to the donated kits provided to our first responders. We demonstrated what is possible when a community comes together. We relied on no government grants or large scale fundraising efforts, and no one profited from this endeavor. And in the end we hope to offer some important and relevant information to our community and beyond.
Update on Cloud Medical testing program
FOR COVID19
⇒ What are the different ways for testing for the virus again?
1. The PCR test uses a nasal swab to collect a sample, detects genetic material that is extremely specific for the virus that causes COVID19.
2. The two types of antibody tests, also called serology tests, detect IgM and IgG antibodies in the blood. The simpler, faster, and more economical version is the fingerprick test (also called a Lateral Flow Assay, or LFA) which many of you had. The more complex version requires a blood draw and provides a quantified antibody result (it gives you an actual number corresponding to the level of antibodies in your blood).
⇒ Do the antibody tests, in general, have sufficient sensitivity to detect known positive COVID pts (who had [+] PCR)? And if so, can we use antibody tests in wide-scale populations to reliably and accurately determine the prevalence of COVID19 exposure without missing too many positives (low false negatives)?
This was a major question that the recent Whitman study in California sought to answer. They collected blood from known symptomatic and [+] PCR patients at varying times following the onset of symptoms (ranging from 1day to over 20+ days after their illness began). They found that the sensitivity of antibody testing increased from 26% to 91% with fingerprick tests (and, similarly, from 18% to 91% with blood draw antibody tests) over the course of the first 3 weeks after symptom onset, with the highest percentage of positives occurring after day 20.
Their results showed that, in their sample, about a quarter of patients developed detectable antibodies in the first week. This rose to over half in the second week, and by the third week over 80% had antibodies.
In our project thusfar we have seen that 100% of those who tested [+] on PCR test over 2 weeks ago had positive antibodies on the fingerprick test and 50% were positive on the blood draw test. So the sensitivity of the fingerprick test appears to be reliable although our sample of PCR positive patients is small and some are still pending. Another unanswered question which we hope we will be able to address is how the populations of most of the studies published to date (which were mostly done on very sick hospitalized patients) compare with an outpatient community-based group like ours.
⇒ What is known about the accuracy of the UCP fingerprick test in comparison to the many other manufacturers of such tests?
The Whitman study showed that UCP was among the most accurate in terms of specificity, indicating a low rate of false positives which according to the authors, “is crucial in low prevalence settings,” such as in non-hospitalized community populations such as ours.
But we have to keep in mind that the results depend on so many things including: whether you were actually exposed to the virus, how severe the exposure was (in terms of viral load), how long ago the exposure occurred in relation to the test date, etc.
⇒ Can the faster, less expensive, more convenient fingerprick test be used as a surrogate a for blood draw antibody test (is there a strong enough correlation between them)?
Although the fingerprick tests we have run thusfar have not missed any positives confirmed by PCR, numerous individuals with positive fingerprick tests have turned out to be negative on blood draw antibody testing.
Initially we were guided by UCP, the manufacturer, to consider any degree of color on the test strip line, no matter how faint, to be positive. However recent testing has revealed that in order to optimize specificity and avoid false positives, only the very dark lines (levels 4 – 6 below) should be considered positive. We are therefore reclassifying all of our data and it may be the case that all but the darkest test strip results will need to be classified as negative.
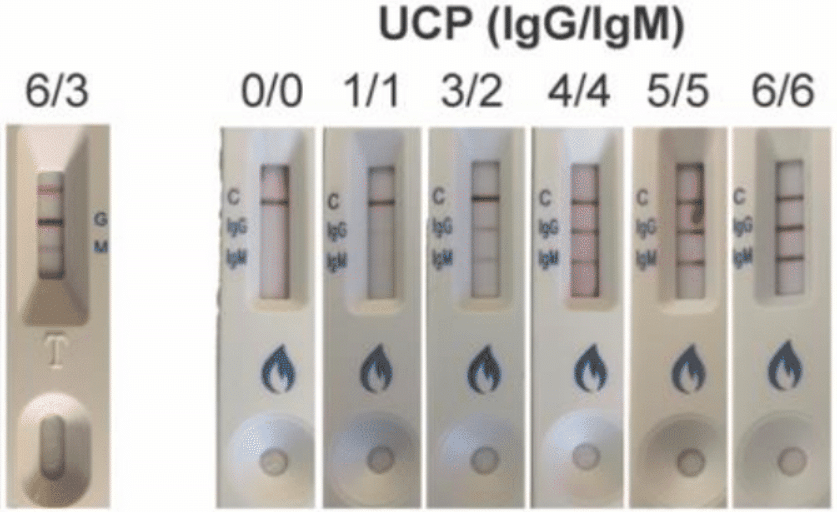
In the samples that we have analyzed thusfar we are seeing inconsistent correlation between the color intensity of fingerprick test and the numeric value of the blood draw antibody. But that may not end up being a relevant issue if we discard all faint and very faint positives. We have had some samples with extremely high antibody levels on the blood test only show up as a level 2 line on the fingerprick. Similarly we have had level 3 fingerprick test results correlate with zero detectable antibodies on the blood test. We are processing all of this data and will keep you updated as new information is obtained.
We will continue to recommend that anyone with any degree of positive result on the fingerprick test be confirmed with a blood draw antibody test.
⇒ Can a negative fingerprick test be used to reliably rule out seroconversion? (can a negative result be trusted as an indicator of non-exposure to the virus?)
An important question on everyone’s mind (and one of the main goals of our testing program) is how to identify those who have been exposed to the virus that cause COVID19 (whether they were ever sick or not), and whether measuring the antibodies which appear during the initial exposure (IgM) and those that appear a bit later and which imply immunity (IgG) can help us do so. The appearance of these antibodies is called “seroconversion”. A positive result on the blood test for either IgM or IgG requires that the level of the antibody rise above 1.0 AU/mL.
Thusfar no one who tested positive on either PCR or on the blood antibody test has come back negative on the fingerprick test. This means that we not seen any false negatives (so the sensitivity of the fingerprick test appears to be high), and at least in our preliminary samples the negatives appear to be true negatives as far as we can tell. However this sample size is still small and many more blood tests are expected to result in the coming week.
⇒ What does a positive IgM fingerprick mean?
Thusfar, of the numerous individuals who have tested positive for IgM on our fingerprick test, none have turned out to have a positive IgM level (above 1.0 AU/mL) on the confirmatory blood test. The Whitman paper also found that the IgM antibody was more variable than IgG. While it is possible that we are simply seeing false positives on the fingerstick, Dr. Michael Lin and others have pointed out that the UCP fingerprick test appears to one of the best tests with respect to the low incidence of false positives.
Again, this discrepancy may disappear once all of the results are reclassified using the 6 level rating system above.
For the time-being, we continue to recommend repeating any positive IgM fingerprick test with a blood draw antibody, and we encourage those patients to take extra precautions since (if the IgM turns out to be a true positive that is confirmed by the blood draw test) it could be an indication of potential contagiousness.
⇒ What does a positive IgG fingerprick mean?
So far we are seeing fewer positive IgG fingerprick results and much lower IgG antibody levels on the blood draw test than for IgM. This is to be expected since IgM rises sooner. However it is also possible that IgG may simply turn out to be a more specific indicator of COVID19 infection (i.e. it may turn out that IgG has less less false positive results than IgM).
If you have a positive IgG, just as with IgM, we recommend confirmatory testing with blood draw.
⇒ What if I have an antibody blood draw test that is “negative” but the number on the report is not zero?
Although the threshold for a “positive” antibody blood draw test result is > 1.0 AU/mL, it is possible that milder cases of COVID19 trigger a lower antibody response which do not rise to the 1.0 threshold. We are seeing significantly higher baseline IgM antibody levels than IgG and are wondering if this could be due to an artifact of prior exposure to other corona viruses. It is also not entirely clear whether the threshold of 1.0 AU/mL to indicate a positive result on the blood test is as optimal a cut-off to use in a community-based outpatient setting, as it appears to be in an inpatient setting. We also wonder whether a titer that is close to (but not above) 1.0, even though not technically considered “positive”, may confer some immunological protection against COVID19 primary infection or re-infection.
We are carefully following research developments and will continue to posts updates on this blog as more information is obtained.
⇒ Does Governor Polis’ recent update make sense?
It seems that our Governor is trying to strike a balance between the medical and socioeconomic realities of the virus.
Based on everything that we know, it is possible to safely move around outside of your home without taking unnecessary risks of exposure and spread, but such risks are dependent upon population density more than anything else. It makes sense that less densely populated communities can loosen restrictions sooner and more easily than downtown Denver, which is why Mayor Hancock is taking a more conservative approach. We encourage all of our members to spend time in the magnificent outdoors but continue practicing social distancing and hand-washing. As much as we can do it safely and methodically, we should take careful steps to begin reviving our economy.
Be well and stay safe out there,
Your Cloud Medical Team

